Abstract
Introduction
Since 2000, phase III randomized controlled trials (RCT) investigated the efficacy of rabbit anti-thymocyte globulins (ATG) in patients following allogeneic stem cell transplantation (allo-SCT). However, comparisons of different ATG formulations do not exist. Our aim was to synthesize all efficacy evidence, enabling a comparison of all available formulations of rabbit ATG in the allo-SCT setting.
Methods
We performed a systematic literature review to identify all available phase III RCTs using EMBASE®, MEDLINE®, MEDLINE® in-process, the Cochrane Library and the website www.clinical-trials.gov. In addition, meeting abstracts (ASH, ASCO, EHA) were screened to include the most up-to-date evidence. Studies were included if they described a phase III RCT among adult patients who received rabbit globulins in the allo-SCT setting and if they reported results of the following end points of interest: chronic graft-versus-host-disease (GVHD, all grades), acute GVHD II-IV and III+IV, transplant-related mortality (TRM), overall survival (OS), incidence of relapse and CMV reactivation.
The actual network meta-analysis (NMA) was performed by using a conventional Bayesian fixed-effect framework and hazard ratios (HR) for most end points while risk ratios (RR) were used for dichotomous outcomes. Analyses were done with the R package netmeta. The most common treatment, cyclosporine and methotrexate, was used as reference treatment.
Results
We identified six phase III RCTs comprising five full publications and one conference abstract between 2001 and 2016, which investigated the following treatment options: 1) Grafalon® (Neovii Biotech, Graefelfing, Germany) consisting of polyclonal IgG obtained from hyperimmune sera of rabbits immunized with the human Jurkat leukemic T-cell line (ATLG), and 2) Thymoglobulin® (Genzyme-Sanofi, Lyon, France), in which immunization was done with human thymocytes recovered from patients undergoing cardiac surgery (Thymo).
The overall population comprised 871 patients (ATLG: n = 610, and Thymo: n = 261). Population per trial ranged from 27 to 126 patients in the ATG arm and from 25 to 128 patients in the non-ATG arm. The median age of patients ranged from 28 to 49 years. More patients were male, except in the ATG arm of one ATLG trial.
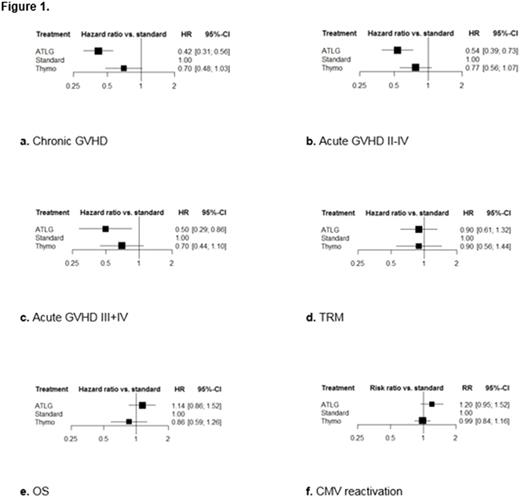
To include all trials within one framework, we had to assume: 1) the relative efficacy of ATG versus cyclosporine and methotrexate was identical to that of tacrolimus and methotrexate, which was used as reference treatment in one ATLG trial; 2) no difference in efficacy due to different graft sources or conditioning regimens; 3) no difference in efficacy due to dosage scheme. Figure 1a-f presents the NMA results of the main end points with HRs/RRs and 95% confidence intervals (CI) versus standard treatment (cyclosporine and methotrexate).
Only ATLG was significantly better than standard treatment in preventing GVHD with a HR of 0.42 (95% CI, 0.31 to 0.56) regarding chronic GVHD, and HRs of 0.54 (95% CI, 0.39 to 0.73) and 0.50 (95% CI, 0.29 to 0.86) regarding acute GVHD II-IV and III+IV. The risk of chronic, acute GVHD II-IV and III+IV was reduced by ATLG with 58%, 46% and 50% versus standard treatment.
Both ATLG and Thymo were at least similarly effective versus standard treatment and showed no difference in relative efficacy regarding TRM with HRs of 0.90 (95% CI, 0.61 to 1.32) and 0.90 (95% CI, 0.56 to 1.44). Considering OS, incidence of relapse and CMV reactivation, the NMA yielded at least similar results of both formulations versus standard treatment, with Thymo tending to be better regarding OS with a HR of 0.86 (95% CI, 0.59 to 1.26) while resulting less clearly in better relative efficacy regarding relapse with a HR of 0.92 (95% CI, 0.68 to 1.24) and CMV reactivation with a RR of 0.99 (95% CI, 0.84 to 1.16).
Conclusions
Our NMA compared all available rabbit ATG formulations and identified ATLG being the best option to prevent chronic and acute GVHD. Both formulations show similar efficacy in TRM while Thymo tends to be better regarding OS. Since randomized head-to-head comparisons are missing, this NMA provides a complete overview of each formulation's relative efficacy.
Kroeger: Neovii: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.